- Wesley Brown

- Mar 5
- 7 min read
Updated: Mar 28
What it took to see atoms
Have you ever looked under a microscope? If you have, you've probably wondered what would happen if you could zoom in forever. Luckily for all of science you weren't the first one to think about this. For many years great scientists wondered what it would look like if we could zoom in far enough to see atoms. They began to ask the question: Why is it so hard to see atoms in the first place? The answer lies in the nature of light itself—specifically, the electromagnetic spectrum. Visible light, which our eyes can detect, has wavelengths ranging from 380 to 750 nanometers. However, atoms are incredibly tiny, measuring just 0.1 nanometers across. This means atoms are over 3,000 times smaller than the wavelengths of visible light, making them impossible to see with conventional light-based methods. If the wavelength of light is much bigger than the object you're trying to see it will just bend around it, making the object invisible. So if we want to see atoms we need to use something with a much smaller wavelength and the best candidate isn't even light, its electrons.

In 1924 French physicist Louis de Broglie worked out that everything, including matter, was sort of wave-like. After de Broglie’s discovery, scientists began tinkering with the idea of using electrons in a microscope. The first obvious problem was that glass lenses, similar to those in a camera or telescope, can't bend electrons the same way they bend light so it would require a different type of lens to focus the electrons. German physicist Hans Busch published a paper about the potential use of electromagnetic lenses to solve this problem. Fortunately, a copy of that paper landed in the lap of Ernst Ruska who was a bright-eyed PhD student at the time (Fig 1. A). Ruska began working on a prototype by coiling up some wire and surrounding it with iron, leaving a hole in the center. Then, he passed a current through the coil which generated a donut shaped magnetic field through the iron and across the gap in the center. To test the lens, he boiled electrons off a tungsten filament, accelerated them through a positively charged anode and directed them into the lens. This basically turned free floating electrons into a beam. Busch's idea worked and Ruska was in complete awe.
By 1931, Ruska had used this design to build the first ever fully functioning electron microscope (Fig 1. B). The microscope’s image was generated when the electron beam struck the sample at its focal point. As long as the sample was no thicker than 100 nanometers at its thickest point, more electrons could pass through the thinner areas compared to the thicker ones. This difference in electron transmission created a detailed electron imprint of the sample, effectively capturing its structure. Then, a second electromagnetic lens would magnify this imprint onto a fluorescent detector Producing the final image. This process is what we call today, transmission electron microscopy (TEM). As spectacular as this achievement was, the early electron microscopes could barely magnify beyond an optical microscope, maxing out at around 1,000x. Ruska and his colleagues had a long way to go before they would be able to see atoms. Determined to improve upon the initial design, Ruska began adding more electromagnetic lenses and by the mid-1930s he had managed to increase the TEM’s max magnification beyond 10,000x. It was producing breathtaking images of bacteria and viruses at a level far surpassing any optical microscope (Fig 1. C & D). The scientific community was astonished and it seemed as though viewing atoms was just a matter of properly calibrating the right number of electromagnetic lenses.

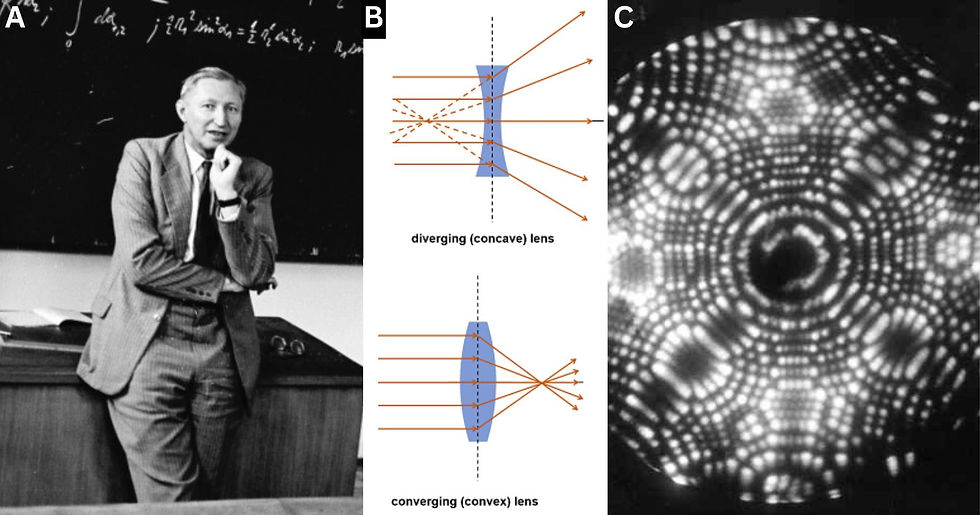
That's when a German physicist named Otto Scherzer published a paper claiming that the TEM was about to hit a brick wall (Fig 2. A). In his seminal paper, Scherzer addressed a fundamental issue of symmetrically spherical lenses known as spherical aberration, which occurs when electrons passing through the edges of a magnetic lens are focused more strongly than those passing through the center. Electrons traveling at different distances from the optical axis of a round lens are focused at different points. Put simply, this makes some of the electrons diverge instead of converge, resulting in a blurred image that limits the resolution of the microscope. The blur starts out at the edges of the image, but inherently gets worse as magnification is increased beyond a certain point. You can actually see this happen with any spherical lens such as those in magnifying glasses. The surprisingly easy solution is to add a diverging lens with the same exact amount of aberration. Since the diverging lens’ aberration is in reverse to your original converging lens, stacking the two will cancel out aberration altogether (Fig 2. B). This is indeed what most advanced lenses use today known as a correcting diverging lens.
So then the TEM just needed it's own version of a correcting diverging lens right? Well, not quite. With magnets this is physically impossible. Every magnet has two poles and all magnetic field lines have to start at one pole and end at the other forming a closed loop. It’s this exact characteristic that causes an electron passing through the electromagnetic lens to always converge, you could never make an electromagnetic lens that diverges electrons. This problem slowed advancements in the TEM significantly just as Scherzer had predicted and in 1955 another microscope beat out the TEM in taking the first ever “image” of atoms (Fig 2. C). This was done using a field ion microscope which functioned by shooting He or Ne atoms at an atomically sharp needle tip. The tip was positively charged, so when gas atoms collided with it, they became ionized and were ejected perpendicular to its surface. This process allowed them to form an impression of the tip's atomic structure. However, this method was very limited as you could only get a sense of the atomic structure and many weren't very impressed by the images.
Over the next four decades, scientists tried boosting the resolution limit of the TEM with various workarounds. The progress was painstakingly slow, but it was progress nonetheless. Perhaps the most clever of all workarounds was the refinements made by Albert Crewe (Fig 3. A). Rather than boiling electrons off the tungsten filament, he opted to pull them off directly using a stronger anode. To enhance the process, he also replaced the filament with a finely sharpened tungsten tip. This provided a narrow beam that was a thousand times brighter than before. Crewe paired these changes with an unlikely partner, the cathode ray tube TV. The cathode ray tube operated by scanning an electron beam across a screen. Crewe realized that if he could scan the sample in small, incremental sections rather than all at once, he could significantly reduce aberrations. By 1970, Crewe had the first image of single atoms taken with the scanning transmission electron microscope (STEM), albeit at ultra low resolution. Some scientists referred to the images as, "splatter in the dark." Unfortunately, even with Crewe’s innovative changes, spherical aberration struck again and the STEM wasn’t able to make it beyond these ultra low resolution images.
During the 1980’s and 90’s a few different versions of probe microscopes, such as the scanning transmission microscope, were created (Fig 3. B). These worked by gliding an incredibly small stylus across the surface of the sample that would detect variations in quantum effects and then map the surface structure of the sample (Fig 3. C). Since they didn't use any lenses they weren't limited by spherical aberration. It was these probe microscopes that gave us the famous orange and yellow image that ended up in almost every 90’s science textbook (Fig 3. D). While that famous image was being printed away, scientists Knut Urban, Max Haider, and Harald Rose were working on one of the craziest ideas yet. You see, Scherzer's theorem stated that spherical aberration was a fundamental issue of symmetrically spherical lenses. Urban, Haider and Rose thought: Well what if you just gave up that symmetry? The obvious issue with this idea is that radial symmetry is one of the most important properties of any lens. If you break the symmetry then you also break the image, but just like Ruska and Crewe before them, they were determined. Their hope was that there would be a small part of this broken image that would be divergent and then they could use that divergent image to correct the aberration of the original lens. Almost like a virtual correcting diverging lens.

They used a cluster of electromagnets known as the hexapole, octopole, and decapole magnets. As the electron beam passed through the center it twisted the 2D image into crazy star-like shapes but the center of the image has a concave bow similar to a diverging lens. The image then passes through a second cluster of magnets returning it to its original shape, but with a slight remnant of the divergence still in its center. This slight remnant would be just enough to reverse the aberration in the opposite direction completely countering the effect. With this method, the group was able to get the TEM resolution down to just 0.13nm! They had achieved what was seemingly impossible. It wasn't long after their breakthrough that Ondrej Krivanek achieved the same for Crewe’s STEM. In modern science, it’s now commonplace to see atoms with such clarity that meaningful analysis at the atomic scale is routine. These innovations have allowed us to make tremendous leaps in materials science, chemical engineering, and numerous other fields. All four received the 2020 Kavli Prize in Nanoscience for their groundbreaking contributions to electron microscopy (Fig 4).







Comments